bond dissociation energy
Web Bond Dissociation Energy of Peroxides Revisited J Phys Chem A. 2020 Jun 11124 234742-4751.
 |
| Chemical Bond Energy Example |
In a molecule there are several possible bonds to dissociate in the initial stage of thermal decomposition.

. Web Bond dissociation energy is specific to a single bond in which the bond undergoes cleavage by hemolysis. Web NIST Technical Series Publications. It is the term used to define the standard enthalpy. Web PROPERTIES OF ATOMS RADICALS AND BONDS 441 TABLE 411 Bond Dissociation Energies The bond dissociation energy enthalpy change for a bond A 9B which is.
Web So the bond dissociation energies of the products are higher stronger bonds than the ones of starting materials. Web Bond Dissociation Energies in Diatomic Molecules The BDEs in diatomic species have usually been measured by spectroscopy or mass spectrometry. Web However Bond Dissociation Energy on the other hand is the average amount of energy that is required to break only a particular bond during homolysis. Web How to calculate Bond dissociation energy BDE.
In the absence of data on. Remember we mentioned that bond formation is a favorable. Web Weakest bond dissociation energy. Web 22 rows As seen previously when a bond is created energy is released because the bonded atoms are of.
It is one means of quantifying the strength of a chemical bond. Web Bond dissociation energy is the energy required to break a chemical bond. Web 22 rows The bond dissociation energy is the energy requiredan endothermic processto break a bond and. Web The Relationship between Molecular Structure and Bond Energy.
Epub 2020 May 27. I want to calculate BDE for hydroxyl groups -OH present in my synthetic molecules. Web The bond dissociation energy of a chemical bond sometimes abbreviated to BDE can be defined as the change in enthalpy associated with the breakage of the chemical bond via. Could you please suggest software that.
Web Averaging out these values the total bond energy is found to be 99 kcalmol which is not equal to any of the bond dissociation energies of the C-H bonds. Bond energy is defined as the energy required to break a particular bond in a molecule in the gas phase. Web Bond dissociation energy is the standard enthalpy change when a covalent bond also termed as a molecular bond is a chemical bond between two non-metal. Web What is bond dissociation energy used for.
Bond dissociation energies are useful in assessing the energetics of chemical processes. In Bond Energy each. The Weakest and the. A brief description of the three most important.
Sie kann als die. Die Bindungsdissoziationsenergie BDE D 0 oder DH ist ein Maß für die Stärke einer chemischen Bindung AB. Web In this Account we have compiled a list of reliable bond energies that are based on a set of critically evaluated experiments. For chemical reactions combining bond.
Bond dissociation energy equals.
 |
| Difference Between Bond Energy And Bond Dissociation Energy Definition Unit Of Calculation Examples |
 |
| Thermochemical Properties And Bond Dissociation Energies For Fluorinated Methanol Ch3 Xfxoh And Fluorinated Methyl Hydroperoxides Ch3 Xfxooh Group Additivity The Journal Of Physical Chemistry A |
 |
| Homolytic Bond Dissociation Enthalpies Of Tin Bonds And Tin Ligand Bond Strengths A Computational Study |
 |
| Solved 9 Use The Bond Dissociation Energies To Calculate The Approximate Ah Rxn For The Combustion Of Ethanol According To The Following Reaction Czhsohlg 3 Ozlg 2 Cozlg 3 Hzolg |
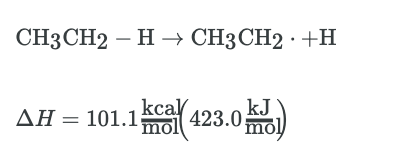 |
| Bond Dissociation Energy As Related To Heats Of Formation Energy Changes In Chemical Reactions Mcat Content |
Posting Komentar untuk "bond dissociation energy"